Carbon is one
of the elements on the Earth. There are two
types of carbon. They are Carbon 12 and Carbon 14. Most of the
carbon atoms are Carbon 12. It has a stable carbon atomic structure and is
non-radioactive.But Carbon 14
isn’t stable and has radioactive carbon isotope.
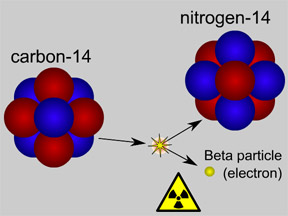
Carbon 14 is
formed after Nitrogen 14 is affected by cosmic rays and solar winds.
Both Carbon
12 and 14 existed in the atmosphere as carbon dioxide. They are absorbed by
plants all alike. Animals eat plants so they absorb Carbon 14 too.
When animals
and plants are alive, although Carbon 14 will change back to nitrogen, they
continue to absorb Carbon 14, so the ratio of both types of carbons in the
bodies is more or less the same as that in the atmosphere.
However,
living things won't be able to absorb Carbon 14 anymore after dying. Carbon 14
inside the bodies will decrease. The longer they die, the less carbon 14 they
have in their bodies.
The
radioactive half-life of Carbon 14 is about 5,730 years, meaning that half of
the Carbon 14 will be converted back to nitrogen every 5,730 years. 5,730 years
later, only 1/4 of the original Carbon 14 will be left while 5,730 more
years later, only 1/8 of the original Carbon 14 will be left.
The ratio of Carbon 14 and 12 in the atmosphere is about 1 to 10 billion.
Therefore, if
a person knows how much Carbon 14 a living thing had when it died, then he/she
can calculate how long it’s been dead.
The best
equipment these days, Accelerator Mass Spectrometer, can test the age of
fossils as far as about 90,000 years ago.
However,
there’s a seriously wrong assumption in the test. Scientists
who believe in evolution assume that there’s no great mutation in the last
billions of years and that there’s no change in the elements in the atmosphere
and cosmic rays. Therefore they assume that the appearance and decline of Carbon 14 are already balanced. They also think that the amount of Carbon 14
now and that billions of years earlier are the same.
The ratio of
carbon 14 will be affected under certain situations. For instance, people began
to burn lots of coals and oil ever since the industrial revolution in 17th
century. This increases the amount of carbon in the atmosphere. But coals
contain less Carbon 14 because they were located underground and were not
affected by cosmic rays.
Therefore,
living things existed after the industrial revolution have a lower ratio of Carbon 14 and Carbon 12. If we don’t know this, then the age calculated by the
equation will be older than the object’s real age.
Another
example is that the amount of carbon 14 has increased after atomic bomb tests
since 1940. Therefore, the age calculated will be younger than the real one.
Moreover, the
strength of geomagnetic field will also affect the amount of Carbon 14. If it
is great, it will protect Carbon 14 from being affected by cosmic rays. It is
confirmed through tests of lava that our geomagnetic field has been decreasing
in the last thousands of years.
In addition,
solar activity and cosmic rays are more intense so the amount of Carbon 14 is
greater than that in the past. Therefore, testing samples thousands of years
earlier will result in thousands or even millions of years in error.
In fact,
diamonds are thought to be heated and under pressure underground for millions
of years. The Carbon 14 inside should be disappeared after millions of years
but scientists found that there is still Carbon 14 inside.
Contemporary
scientist discovered that the half-life of radioactive samples in the labs
changes instantly as the Earth is hit by the solar wind. Therefore, the assumption
saying that radioactive half-life is constant is completely broken.



支持,好文章。
ReplyDelete值得細閱。
Science could not be fully convinced. Deeply thanks for sharing such a scientific article.
ReplyDeleteGreat!!deeply appreciate
ReplyDeletewhy not scientist told us the fact, but Church?
ReplyDeleteGreat support thees useful information!
Wa......its so surprising!!!
ReplyDeletewhy no news telling us about this?.....poor...>"<
Thanks for sharing~!! Which really help us to know more!
ReplyDeletethx for sharing
ReplyDelete